Ensuring widespread distribution, usage and effectiveness of the COVID-19 vaccine in South Dakota will be challenging, health experts say, due to the rural nature of the state, the difficulty in storing and distributing doses of the vaccine and concerns that some people will be reluctant to be vaccinated.
South Dakota will soon have thousands of the first round of new COVID-19 vaccines to distribute to front-line healthcare workers as part of what many see as the world’s next steps toward an end to the deadly pandemic.
Two vaccines, one made by Pfizer and one made by Moderna, are currently on the cusp of approval for use in the U.S. Both vaccines require very cold storage before use, require two doses to achieve their full effect, and are based on a technology never before used to make a vaccine.
Medical experts estimate that between 60% and 90% of a population needs to be vaccinated or otherwise immunized against COVID-19 to effectively protect people from the virus.
The sooner a majority of South Dakotans are immunized against the coronavirus, the sooner the pandemic will end, said Dr. Jeremy Caulwels, senior vice president for quality at Sanford Health.
“That is really what it takes to prevent the spread of an illness within a population,” he said. “That’s very much our goal to generate that kind of immunity in our populations and in our kids and our health-care workers.”
The cold storage requirement may prove to be a big hurdle for COVID-19 vaccination in South Dakota. Few hospitals in the state have freezers capable of storing the Pfizer vaccine, which must be kept at roughly minus-158 degrees Fahrenheit, said Dana Darger, director of pharmacy for Monument Health Rapid City Hospital. Both the Moderna and Pfizer vaccines also have relatively short shelf lives, he said.

“It’s only good for five days in a refrigerator. And it’s only good for six hours after you take it out of the refrigerator,” Darger said. “There are five doses in a vial, so we’re trying to figure out how we get vials where we need them and that we can use them in a reasonable amount of time to optimize those five doses. It’s not undoable, but it does present some challenges.”
Ensuring that South Dakotans get both doses of either the Moderna or Pfizer vaccine may also be challenging. Rural residents, in particular, may have trouble getting their second dose because they likely will have to travel significant distances.
“We need to make sure that we understand the flow and make sure that people understand this is a two-dose vaccine,” said Caulwels. “The first dose doesn’t quite cut the mustard. You have got to get two (doses) in order to be protected.”
As South Dakota hospitals wrestle with how to get people vaccinated, the U.S. government will be struggling with how to get vaccines to hospitals. The COVID-19 vaccine distribution effort will be the most extensive such program in the nation’s history. Throughout 2021, the federal government will be buying and distributing hundreds of millions of doses of multiple vaccines, each with separate dosage, storage and transportation requirements.
Nationwide, public health experts have started to warn that confusion, vaccine hesitancy and anti-vaccine misinformation being spread on social media and elsewhere likely will hamper efforts to immunize large enough populations to slow the spread of COVID-19.
“There will absolutely be confusion. And it’s going to be incredibly important that at the federal level, there is a single, clear message about what steps they are taking and what steps come next,” said Alta Charo, a professor of law and bioethics at the University of Wisconsin who has worked extensively with public vaccination efforts.
Anti-vaccine sentiment in South Dakota and across the country is threatening to derail efforts to end the pandemic through widespread immunization with vaccines.
The number of Americans who say they are willing to take a COVID-19 vaccine has been declining steadily since the spring. Polls conducted by the Pew Research Center and Gallup in late September found that only about half of Americans would be willing to take a COVID-19 vaccine, which was down from May when around two-thirds of people polled said they were likely to take a vaccine.
In the 2020 South Dakota legislative session, lawmakers debated a bill that would have eliminated vaccine requirements for children being enrolled at South Dakota public schools. In sometimes emotional testimony, the bill was backed in part by Health Freedom South Dakota, a non-profit organization created to advocate against vaccine mandates.
Ultimately, legislators killed the bill. Health Freedom South Dakota did not respond to interview requests from News Watch.
Still, one piece of data suggests that South Dakotans have a better track record of taking recommended vaccinations than other states. Between 2013 and 2019, South Dakotans over the age of 65 had been consistently more likely to get a flu vaccine than Americans over 65 as a whole, according to data collected by the Centers for Medicare and Medicaid Services.
Vaccine distribution will be complicated
The U.S. government has signed contracts with several manufacturers to make hundreds of millions of vaccine doses before testing on them is complete. The idea is to have vaccine doses ready to distribute more quickly when they are approved for use. Because the federal government has already bought the vaccines, state governments will take charge of distribution.
As vaccine doses become available, federal officials will allocate them to states based on population. Once federal officials determine how many doses of each vaccine a state will get, the state government will decide who will be first in line for the injections.
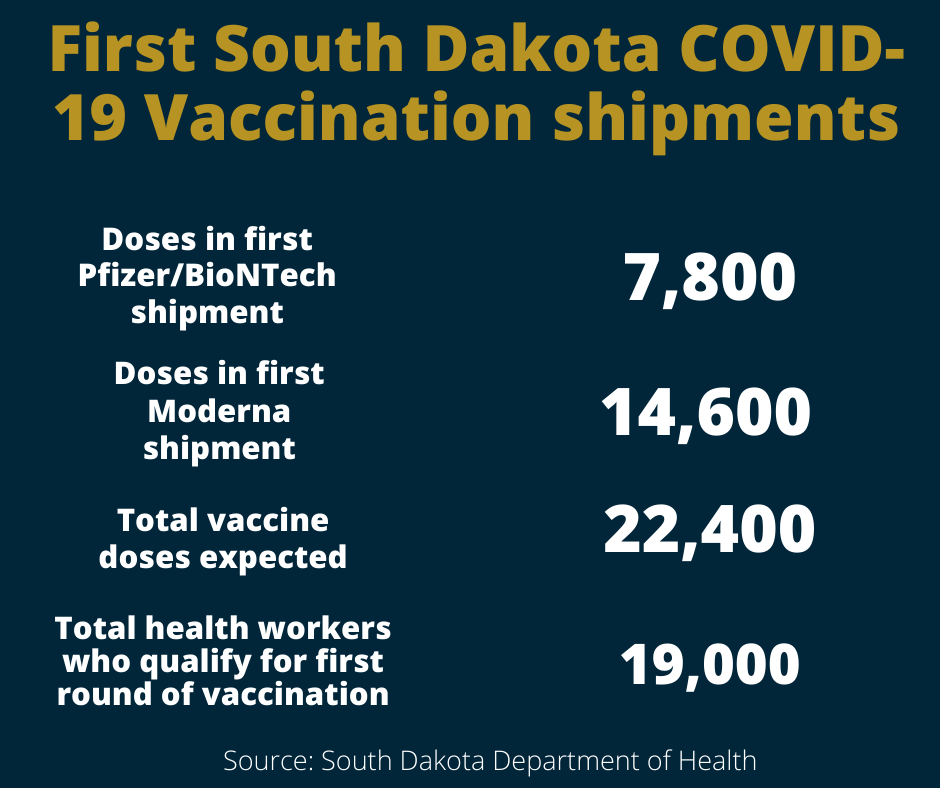
South Dakota was slated to receive 7,800 doses of Pfizer’s vaccine, the first COVID-19 vaccine to be given an emergency use authorization from the Food and Drug Administration, as soon as it gets FDA approval.
The first shipment of vaccines was expected to arrive in South Dakota within days of the FDA authorization, state Department of Health Secretary Kim Malsam-Rysdon said during a Dec. 9 news conference. Pfizer will send another set of 7,800 doses within about three weeks to provide second doses to people who got the first round of shots, she said.
Moderna is the maker of the second vaccine slated for Emergency Use Authorization. South Dakota is expected to receive 14,600 doses of that vaccine if it is authorized as anticipated on Dec. 17. Moderna would then send a second round of doses about three weeks after the first batch arrives to supply the second round of shots.
In all, the first couple of vaccine shipments from Pfizer and Moderna should give the state enough doses to immunize 22,400 people. There should be enough vaccines for the roughly 19,000 South Dakotans who qualify as frontline healthcare workers or long-term care workers, Malsam-Rysdon said.
According to the state Department of Health, the general public is not expected to have ready access to either Moderna’s or Pfizer’s vaccine until well into 2021.
The Centers for Disease Control and Prevention has created a “playbook” for state health officials to follow when distributing COVID-19 vaccines. South Dakota’s vaccine distribution plan calls for a three-phased vaccine rollout.
The first phase of vaccine distribution could begin as early as Dec. 15. Vaccine use will be restricted to doctors and nurses who take care of hospitalized COVID-19 patients, staff at nursing homes and assisted-living centers and others most vulnerable to infection. The vaccines will only be available at larger healthcare facilities.
Nursing home and assisted-living center residents also will be able to receive immunizations during the first phase of the rollout as the supply of vaccines begins to increase, according to the state vaccination plan.
Phase two of the vaccine rollout will begin slowly as vaccine production ramps up. The supply of vaccines should be enough to accommodate anyone who wants to get the shot. Doses of COVID-19 vaccines will eventually be made available in smaller, more rural clinics and, potentially, at drive-through locations. Healthcare providers will be able to charge an administration fee for administering the vaccines but they won’t be able to charge for the vaccines themselves, according to the Department of Health.
During phase three of the vaccine rollout, public health officials anticipate having plenty of vaccine doses and expect slower demand for them. The Department of Health plans to identify parts of the state that saw low rates of vaccination and hold special vaccination clinics there.
To speed up the vaccination process in phases two and three, students and teachers in nursing and pharmacy programs at South Dakota universities and the Sanford USD School of Medicine have volunteered to be trained on how to administer COVID-19 vaccines, Caulwels said.

“It has been remarkable,” Caulwels said. “Everybody is coming together very nicely to say, ‘If you need help to get this vaccine to more people, we will be there to help out.’”
Public confidence critical to success
The extended vaccine rollout will help healthcare providers determine how best to provide vaccines to those who need it, said Dr. David Basel, vice president for clinical quality at Avera Health. It will also give public health officials and healthcare providers some extra time to shore up declining public confidence in COVID-19 vaccines.
“We’re hopeful that when the actual data gets released and can be peer-reviewed, that it will reassure the general public and turn that tide,” Basel said.
Public polling on Americans’ attitudes toward COVID-19 has revealed some troubling trends.
A recent survey conducted by the medical news website STAT and The Harris Poll found that as many as 78% of Americans felt that politics had played a bigger role than science in creating new COVID-19 vaccines. Concerns over vaccine safety also were bi-partisan. Nearly 85% of Democrats and roughly 80% of Republicans said they would be concerned about a vaccine’s safety if it was approved too quickly.
The nature of both the Moderna and Pfizer vaccines may make them safer than other vaccines, said Dr. Benjamin Aaker, an Avera emergency medicine doctor who is president of the South Dakota State Medical Association.

"We believe that the benefits of the vaccine in terms of your own health and in terms of the health of the population in general, and in terms of the economy, far outweigh the risk of taking it." -- Benjamin Aaker, president of the South Dakota State Medical Association
Unlike other vaccines, the Pfizer and Moderna vaccines do not use a live virus as part of their manufacturing process. Instead, both vaccines use a molecule called synthetic messenger RNA that was built in a lab using the COVID-19 virus’ gene sequence as a blueprint. Chinese scientists published the SARS-CoV-II gene sequence on the internet on Jan. 10.
Messenger RNA is a naturally occurring substance used with the human body to tell individual cells what types of proteins to make. Proteins are one of the basic building blocks upon which all life is built and play an important role in the human immune system.
Proteins are also an essential piece of the SARS-CoV-II virus, which causes COVID-19. Proteins form the corona of spikes around the virus that gives the coronavirus its name. The spikes attach to healthy cells and allow the virus to hijack the cells and force them to make copies of the virus.
Both the Pfizer and Moderna vaccines use a synthesized version of messenger RNA that, when injected into someone’s upper arm, instructs nearby cells to temporarily produce a protein that is identical to a protein used by the coronavirus.
Once the fake coronavirus protein makes its way into the bloodstream, it activates the body’s immune system. The immune system then goes to war against the harmless protein and, in the process, learns how to recognize and destroy the SARS-CoV-II virus without ever actually being at risk.
Both vaccines can provoke strong immune reactions, especially after the second dose. Documented reactions include fatigue, mild fever and muscle stiffness for about a day after the second injection.
“You see news about severe side effects, well, severe side effects in the study were that around 9% of people had fatigue. Fatigue is something that is a side effect, but I call that minor when you compare it to getting heart damage from the coronavirus,” Aaker said.
Still, Aaker said, both the Pfizer and Moderna vaccines have been shown to be safe for most people. Late-stage trials also showed that both vaccines were nearly 95% effective at preventing trial participants from developing severe COVID-19 symptoms, he said.
“The vaccine is safe to take but it is not without side effects, as we know from studies. And once it is administered to more people, we will know more,” Aaker said. “But we believe that the benefits of the vaccine in terms of your own health and in terms of the health of the population in general, and in terms of the economy, far outweigh the risk of taking it.”



